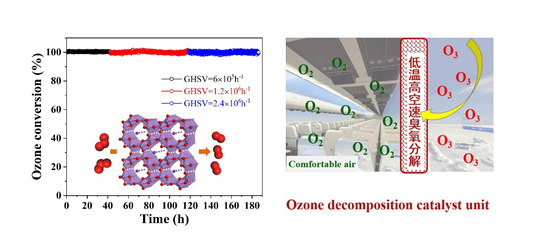
We have developed a series of Mn-based catalysts for ozone decomposition. At room temperature and space velocity of 600,000 h-1, the catalysts can totally decompose 40 ppm ozone and 100 ppb ozone at relative humidity of 45% and 90%, respectively. The activity and moisture resistance of the catalyst were significantly improved by modification with transition metals or noble metals, and the activity extended to the low temperature range under space velocity of 2,400,000 h-1. Physical and chemical characterization showed that the Mn2+ and Mn3+ (oxygen vacancy) of the catalysts was the active site for ozone decomposition, and its content determined the catalytic activity for ozone decomposition; the deactivation mechanism under different conditions was revealed: in the presence of water vapor, the competitive adsorption of ozone molecules and water molecules led to deactivation of the catalyst; under dry conditions, the oxygen atom did not rapidly desorb and poisoned oxygen vacancies, leading to deactivation of the catalysts. Quantum chemistry calculations revealed the existence of different types of oxygen vacancies in the catalysts, which explained the difference in activity, and pointed the way toward developing highly efficient catalysts. The catalytic materials have been successfully used in personal protective products, air purifiers, fresh air systems and functional coatings, providing technical means for eliminating ozone in semi-enclosed spaces and the atmospheric environment. In the future, the technology can be used to remove the ozone in aircraft cabins, and to decompose low-concentration ozone in the atmosphere environment by coating catalysts on the radiators of motor vehicles or the outer surface of buildings. (Appl. Catal., B, 2017, 201, 503; Environ. Sci. Technol., 2018, 52,12685; Environ. Sci. Technol., 2019, 53, 10871)

9. Li Yang, Jinzhu Ma*, Xiaotong Li, Changbin Zhang, Hong He, “Enhancing oxygen vacancies of Ce-OMS-2 via optimized hydrothermal conditions to improve ozone decomposition”, Ind. Eng. Chem. Res., 59, (2020) 118-128.
8. Xiaotong Li, Jinzhu Ma*, Changbin Zhang, Runduo Zhang, Hong He, “Detrimental role of residual surface acid ions on ozone decomposition over Ce-modified γ-MnO2 under humid conditions”, J. Environ. Sci., 91 (2020) 43-53.
7. Li Yang, Jinzhu Ma*, Xiaotong Li, Guangzhi He, Changbin Zhang, Hong He, “Tuning the fill percentage in the hydrothermal synthesis process to increase catalyst performance for ozone decomposition”, J. Environ. Sci., 87, (2020) 60-70.
6. Hua Deng, Shunyu Kang, Jinzhu Ma*, Lian Wang, Changbin Zhang, Hong He, “Role of structural defects in MnOx promoted by Ag doping in the catalytic combustion of volatile organic compounds and ambient decomposition of O3”, Environ. Sci. Technol., 53, (2019) 10871-10879.
5. Xiaotong Li, Jinzhu Ma*, Changbin Zhang, Runduo Zhang, Hong He, “Facile synthesis of Ag modified manganese oxide for effective catalytic ozone decomposition”, J. Environ. Sci., 80, (2019), 159-168.
4. Xiaotong Li, Jinzhu Ma*, Li Yang, Guangzhi He, Changbin Zhang, Runduo Zhang, Hong He, “Oxygen vacancies induced by transition metal doping in g‑MnO2 for highly efficient ozone decomposition”, Environ. Sci. Technol., 52, (2018) 12685-12696.
3. Jinzhu Ma, Caixia Wang, Hong He*, “Transition metal doped cryptomelane-type manganese oxide catalysts for ozone decomposition”, Appl. Catal. B, 201, (2017) 503-510.
2. Caixia Wang, Jinzhu Ma*, Fudong Liu, Hong He, Runduo Zhang, “The effects of Mn2+ precursors on the structure and ozone decomposition activity of cryptomelane-type manganese oxide (OMS-2) catalysts”, J. Phys. Chem. C, 119, (2015) 23119-23126.
1. Zhihua Lian, Jinzhu Ma, Hong He*, “Decomposition of high-level ozone under high humidity over Mn-Fe catalyst: The influence of iron precursors”, Catal. Commun., 59, (2015) 156-160.
Group of Environmental Catalysis and Green Chemistry

You are the 1854288th visitor
Copyright Professor Hong He's Group 2019