Secondary organic aerosols (SOA) are an important part of the fine particles (PM2.5) in urban and suburban atmospheres. The composition and formation process of SOA are very complicated. The formation mechanism of SOA in highly complex pollution needs to be further investigated.
1. Experimental System
1.1. Brief introduction to 30 m3 indoor smog chamber
The smog chamber system, as an effective method to study and evaluate photochemical reaction mechanisms, plays an important role in understanding and solving local, regional and global scale air pollution issues. The 30 m3 indoor smog chamber at the Chinese Academy of Sciences Eco-Environment Research Center (RCEES-CAS) mainly consists of a zero-air generation system, Teflon reactor system, light source system, temperature control system, sampling and control system and detection system. The Teflon reactor is a cuboid reactor (3.0 m (length)×2.5 m (width)×4.0 m (height)) with a surface-to-volume ratio of 1.97 m-1. It is irradiated by 120 UV lamps (Philips) with peak intensity at 365 nm, providing a maximum NO2 photolysis rate of 0.55 min-1. The interior is coated with 125 μm-thick FEP100 film (DuPont™, US) and the chamber is located in a temperature-controlled room in which the temperature (T) and relative humidity (RH) can be controlled mechanically. This RCEES-CAS chamber facility is designed to study the formation mechanisms of O3 and secondary aerosols under complex pollution conditions.
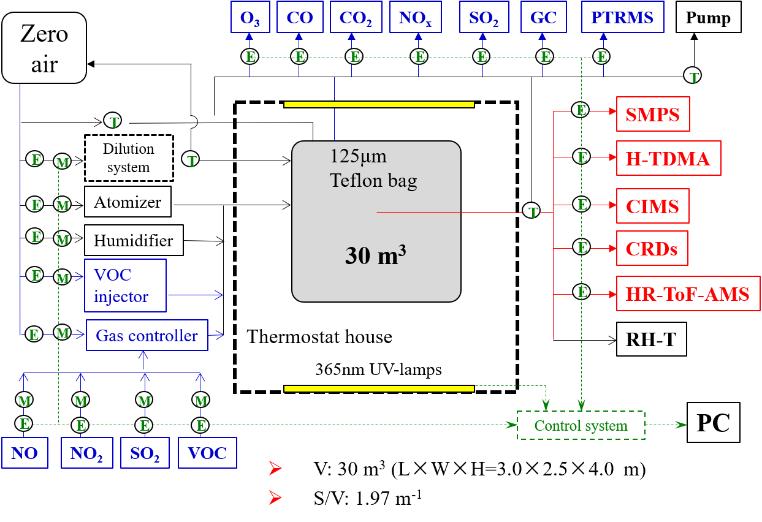
RCEES-CAS 30 m3 indoor smog chamber experimental system
1.2. Oxidation Flow Reaction (OFR) system
Following the design of the potential aerosol mass (PAM) reactor introduced by Kang, two steel cylinder reactors constituting a twin-OFR were used to implement the experiment. The volume of each cylinder reactor is about 15 L. OH radicals were generated in the one reactor by the photolysis of O3 initiated by 254 nm UV light and subsequent reaction with H2O. Due to the very high concentrations of OH radical in the reactor, the OFR can simulate the aging process of aerosol in days or weeks. In the field observation experiment, the air flow was switched between the two reactors. The OFR experimental system was used to study the SOA formation and aging process, as well as the SOA formation potential of ambient air (JES, 2016, 39, 52).
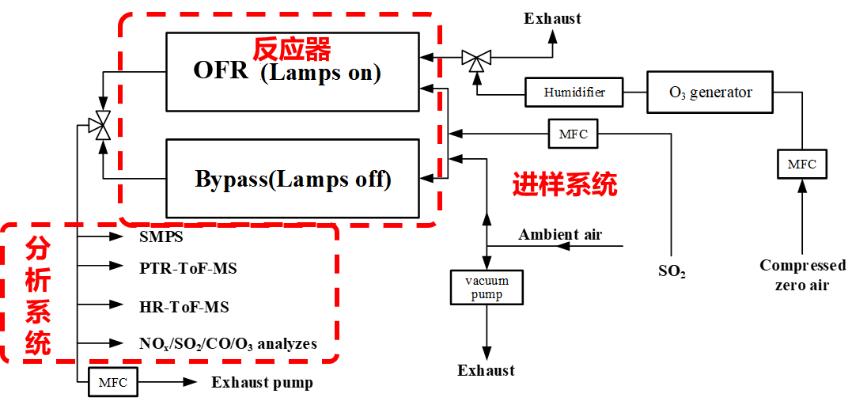
Dual oxidation flow reactor (OFR) system
2. The effects of complex air pollution conditions on SOA formation
2.1. The effects of mineral oxides on SOA yield
The effect of Al2O3 seed particles on SOA yield in m-xylene and α-pinene photooxidation is not clear. However, introducing a high concentration of Al2O3 seed particles will increase the particle number concentration but decrease the particle size markedly. The decrease of SOA size will prolong the atmospheric lifetime of the particles, aggravate the human health risk, and simultaneously change its extinction characteristics (AE, 2013, 77, 781; Sci. China-Earth Sci., 2015, 58(2), 245). Under complex air pollution conditions, the synergistic effects between pollutants can also enhance the oxidation capacity and further change the dependence of secondary aerosol formation on precursors. For example, in the presence of Al2O3 seed particles, SOA formation can be higher under high-NOx conditions than low-NOx conditions (Sci. China-Chem., 2015, 58(9), 1426).
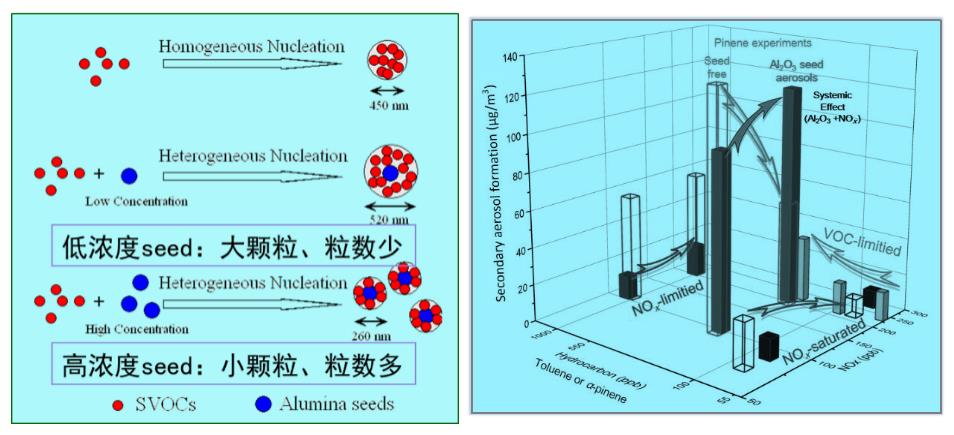
The effects of mineral seed aerosols on secondary aerosol formation
2.2. The effect of inorganic seed particles on SOA aging
SOA formation was simulated in the smog chamber under different seed particle conditions, and it was found that SOA yield decreased significantly in the presence of iron sulfate seed particles (AE, 2012, 55, 26). Reactive oxidant species (ROS) were generated in aerosol surface water in a Fenton-like reaction between iron ions and peroxides during the redox process. These ROS further oxidized the generated SOA, and therefore caused the oxidation degree of SOA to increase and simultaneously generated some organic products with high volatility, which partitioned back to the gas phase and resulted in lower SOA yield (EP, 2014, 193: 88). The significance of these two effects, i.e. decreased SOA yield and increased SOA oxidation degree, was found to be dependent on the chemical properties of SOA generated from different oxidation systems (Chu et al., 2017. Sci. Rep. 7: 40311). These results help to explain the phenomenon that the oxidation degree of SOA in laboratory simulation was generally lower than in field observations. The presence of inorganic seed particles could also affect the hygroscopicity of the generated SOA, and influence its contribution to haze pollution (JES, 2014, 26 (1): 129).

Suppression effect and mechanism of ferric sulfate on SOA formation
2.3. Effect of co-existing inorganic gases on SOA formation
The synergistic effects between inorganic and organic precursors in secondary aerosol formation were studied. SO2 and NH3 were found to make important contributions to the formation of both secondary inorganic aerosol and SOA. Under NH3-poor conditions, SO2 increased SOA formation mainly due to the acid-catalytic effect of its acid aerosol products, which enhanced oligomer formation, while under NH3-rich conditions, SO2 increased the formation of nitrogen-containing organics and organic sulfate and therefore enhanced SOA formation. Meanwhile, the uptake of small, highly oxidized molecules was also enhanced, which also increased the oxidation state of the SOA. Similar effects of SO2 and NH3 were found on SOA formation from a single VOC (i.e. single aromatic hydrocarbon), specific emissions (i.e. vehicle fuel evaporation), and oxygenated volatile organic compounds (i.e. methoxyphenols (one representative tracer of biomass combustion emissions) and some oxygenated intermediate species (i.e. unsaturated ethers and esters)) (ACP, 2016, 16(22), 14219; ACP, 2019, 19, 8063; EST, 2019, 53 (15), 8845; ACP, 2019, 19, 2687; ACP, 2019, 19, 2001; AE, 2019, 207, 30). Under the compound pollution conditions of SO2 (coal-fired flue gas) and NH3 (agricultural non-point source and traffic emissions), the contribution of some emission sources (such as vehicle evaporative emissions, which contributed 0.49 ± 0.04 Tg yr-1 SOA formation) could not be ignored (AE, 2019, 201, 101; ACP, 2019, 19, 8063).
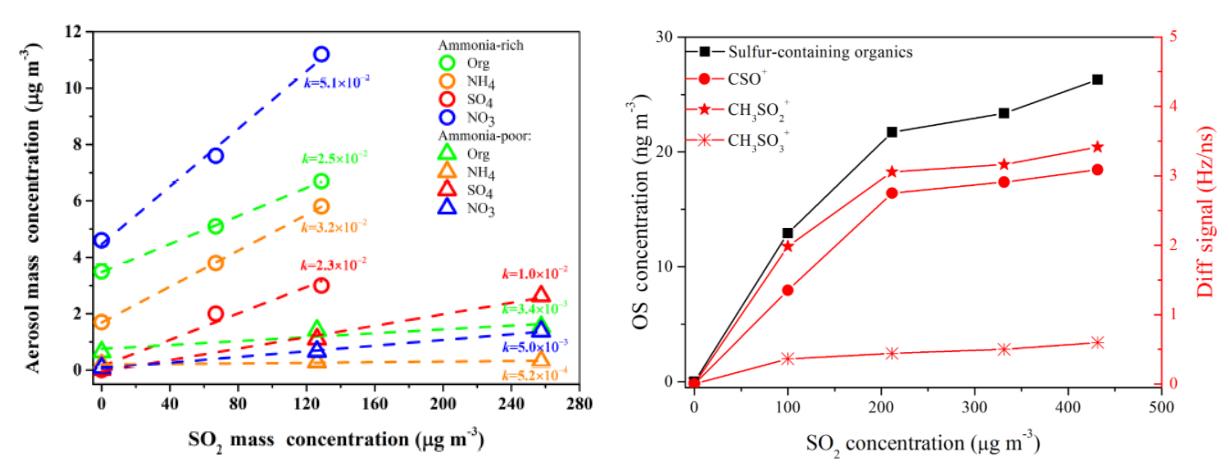
Co-existing SO2 enhanced secondary aerosol formation
3. Oxidation mechanisms in SOA formation
3.1. At low initial precursor concentrations, gas-phase intermediates would be converted into advanced oxidation products more readily, with LVOCs being the main products. Also, the SOA yield at low concentrations is significantly higher than that predicted from the yield curve obtained at high concentrations. This might be one of the reasons that current model predictions generally underestimate the SOA concentration observed in field observations (JES, 2019, 79, 256).
3.2. Under complex air pollution conditions, the formation of organic sulfates and the uptake of small molecules with higher oxidation products were proved to be the crucial factors promoting the increase in the oxidation state of SOA with SO2 concentration. Moreover, the competition between H2O and SO2 in reacting with the stabilized Criegee intermediate (sCI) also exhibited a profound effect on SOA yield and composition during the ozonolysis of unsaturated oxygenated volatile organic compounds. (EST, 2019, 53 (15), 8845-8853;ACP, 2019, 19, 2687-2700).

Deep oxidation of VOCs led to additional SOA formation
4. Field observation study of SOA
4.1. To best represent complex air pollution conditions, SOA formation from ambient air was investigated using an oxidation flow reactor (OFR) with high concentrations of OH radicals (JES, 2016, 39, 52). The SOA formation potential increased significantly with the increase of ambient PM2.5 concentration during the observation in Beijing. Due to the higher concentrations of VOCs, the OA enhancement in Beijing was much higher than that of developed countries under different environmental conditions. The optimum ambient exposure time, which is the aging time equivalent to atmospheric oxidation (with similar OH exposure) associated with peak SOA formation, was about 3 days in this study. Fragmentation did not dominate in the oxidation of OA, and did not result in negative OA enhancement on highly polluted days compared to relatively clean days with similar exposure time. These results suggested that under typical ambient conditions, high concentrations of VOC precursors might contribute to sustained organic aerosol growth and long duration haze events in Beijing (EST, 2018, 52 (12): 6834).

SOA formation from ambient air in Beijing under different pollution levels
4.2. A case study in the southern suburbs of Beijing in summertime showed that organic aerosol (OA) was the dominant chemical composition. The diurnal variations of elemental ratios and OA with different oxidation states were a combined result of photochemical and aqueous-phase processes. Our results also showed that SOA was the dominant species, which increased with the pollution level (Chemosphere, 2020, 247, 125918).
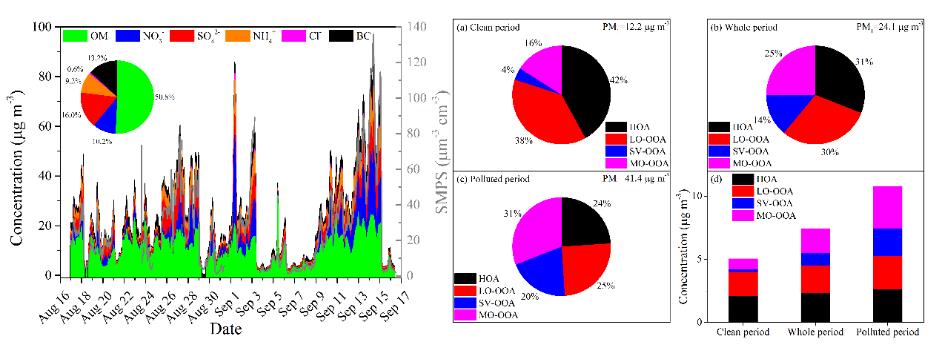
Characteristics of summertime PM1 and OA with different oxidation states in a southern suburb of Beijing

19) Tianzeng Chen, Jun Liu, Yongchun Liu, Qingxin Ma*, Yanli Ge, Cheng Zhong, Haotian Jiang, Biwu Chu, Peng Zhang, Jinzhu Ma, Pengfei Liu, Yafei Wang, Yujing Mu, Hong He, "Chemical characterization of submicron aerosol in summertime Beijing: a case study in southern suburbs in 2018", Chemosphere, 247, (2020) 125918.
18) Peng Zhang, Tianzeng Chen, Jun Liu, Changgeng Liu, Jinzhu Ma, Qingxin Ma, Biwu Chu, Hong He*, “Relative humidity, and seed acidity on secondary organic aerosol formation in the ozonolysis of butyl vinyl ether”, Environ. Sci. Technol., 53, (2019) 8845-8853.
17) Changgeng Liu, Yongchun Liu*, Tianzeng Chen, Jun Liu, Hong He*, “Rate constant and secondary organic aerosol formation from the gas-phase reaction of eugenol with hydroxyl radicals”, Atmos. Chem. Phys., 19, (2019) 2001-2013.
16) Changgeng Liu, Tianzeng Chen, Yongchun Liu*, Jun Liu, Hong He*, Peng Zhang, “Enhancement of secondary organic aerosol formation and its oxidation state by SO2 during photooxidation of 2-methoxyphenol”, Atmos. Chem. Phys., 19, (2019) 2687-2700.
15) Changgeng Liu, Jun Liu, Yongchun Liu*, Tianzeng Chen, Hong He*, “Secondary organic aerosol formation from the OH-initiated oxidation of guaiacol under different experimental conditions”, Atmos. Environ., 207, (2019) 30-37.
14) Tianzeng Chen, Yongchun Liu, Qingxin Ma*, Biwu Chu, Peng Zhang, Changgeng Liu, Jun Liu, Hong He*, “Significant source of secondary aerosol: formation from gasoline evaporative emissions in the presence of SO2 and NH3”, Atmos. Chem. Phys., 19, (2019), 8063-8081.
13) Tianzeng Chen, Yongchun Liu*, Changgeng Liu, Jun Liu, Biwu Chu, Hong He*, “Important role of aromatic hydrocarbons in SOA formation from unburned gasoline vapor”, Atmos. Environ., 201, (2019), 101-109.
12) Tianzeng Chen, Yongchun Liu*, Biwu Chu, Changgeng Liu, Jun Liu, Yanli Ge, Qingxin Ma, Jinzhu Ma, Hong He*, “Differences of the oxidation process and secondary organic aerosol formation at low and high precursor concentrations”, J. Environ. Sci., 79, (2019), 256-263.
11) 刘俊,楚碧武*,贺泓,“北京市二次有机气溶胶生成潜势的日变化规律”,环境科学,39 (2), (2018) 2505-2511.
10) Jun Liu, Biwu Chu*, Tianzeng Chen, Changgeng Liu, Ling Wang, Xiaolei Bao, Hong He*, “Secondary organic aerosol formation from ambient air at an urban site in Beijing: Effects of OH exposure and precursor concentrations”, Environ. Sci. Technol., 52, (2018) 6834-6841.
9) 陈天增,葛艳丽,刘永春*,贺泓,“我国机动车排放VOCs 及其大气环境影响”,环境科学,39 (2), (2018) 478-492.
8) Jinzhu Ma, Biwu Chu, Jun Liu, Yongchun Liu, Hongxing Zhang, Hong He*, “NOX promotion of SO2 conversion to sulfate: An important mechanism for the occurrence of heavy haze during winter in Beijing”, Environ. Pollut., 233, (2018) 662-669.
7) Biwu Chu, John Liggio, Yongchun Liu, Hong He*, Hideto Takekawa, Shao-Meng Li, Jiming Hao, “Influence of metal-mediated aerosol-phase oxidation on secondary organic aerosol formation from the ozonolysis and OH-oxidation of α-pinene”, Sci. Rep., 6, (2017) 40311
6) Biwu Chu, Xiao Zhang, Yongchun Liu, Hong He*, Yele Sun, Jingkun Jiang, Junhua Li, Jiming Hao, “Synergetic formation of secondary inorganic and organic aerosol: effect of SO2 and NH3 on particle formation and growth”, Atmos. Chem. Phys., 16, (2016) 14219-14230.
5) Biwu Chu, Yongchun Liu, Qingxin Ma, Jinzhu Ma, Hong He*, Gang Wang, Shuiyuan Cheng, Xinming Wang, “Distinct potential aerosol masses under different scenarios of transport at a suburban site of Beijing”, J. Environ. Sci., 39, (2016) 52-61.
4) Biwu Chu, Tengyu Liu, Xiao Zhang, Yongchun Liu, Qingxin Ma, Jinzhu Ma, Hong He*, Xinming Wang, Junhua Li, Jiming Hao, “Secondary aerosol formation and oxidation capacity in photooxidation in the presence of Al2O3 seed particles and SO2”, Sci. China-Chem., 58 (9), (2015) 1426-1434.
3) Biwu Chu, Yongchun Liu, Junhua Li, Hideto Takekawa, John Liggio, Shaomeng Li, Jingkun Jiang, Jiming Hao, Hong He*, “Decreasing effect and mechanism of FeSO4 seed particles on secondary organic aerosol in a-pinene photooxidation”, Environ. Pollut., 193, (2014) 88-93.
2) Biwu Chu, Kun Wang, Hideto Takekawa, Junhua Li, Wei Zhou, Jingkun Jiang, Qingxin Ma, Hong He, Jinming Hao*, “Hygroscopicity of particles generated from photooxidation of α-pinene under different oxidation conditions in the presence of sulfate seed aerosols”, J. Environ. Sci., 26, (2014) 129-139.
1) Chang Liu, Biwu Chu, Yongchun Liu*, Qingxin Ma, Jinzhu Ma, Hong He*, Junhua Li, Jiming Hao, “Effect of mineral dust on secondary organic aerosol yield and aerosol size in a-pinene/NOx photo-oxidation”, Atmos. Environ., 77, (2013) 781-789.
Group of Environmental Catalysis and Green Chemistry

You are the 1854288th visitor
Copyright Professor Hong He's Group 2019