Heterogeneous reaction processes on the surface of atmospheric particles will not only affect the source/sink balance of gaseous pollutants, but also change the chemical composition and properties of particles (hygroscopicity and extinction properties, etc.), which play an important role in the formation of haze. However, it is not clear whether a new mechanism exists in the heterogeneous reaction of particles under the conditions of complex air pollution. Therefore, it is urgent to study the interactions and synergistic effects of various pollutants in heterogeneous reactions in the atmosphere, reveal the formation mechanism of secondary particles under the conditions of complex air pollution, and provide a scientific basis for clarifying the causes of complex air pollution in China.
1. Research progress in the research on synergistic effects in heterogeneous reactions
1.1. It was found for the first time that coexisting NOx can greatly promote the conversion of SO2 and sulfite to sulfate. The critical role of NO2 and mineral oxides jointly catalyzing the activation of molecular oxygen and promoting the formation of sulfate has been revealed (JPCA, 2008, 112, 6630; PCCP, 2012, 14 (5), 1668; PCCP, 2013, 15, 19196);
1.2. It was further confirmed that SO2 can initiate the conversion of NO2 to HONO on the MgO surface, which may be a potential source of HONO in the atmosphere (EST, 2017, 51, 3767);
1.3. The interaction mechanism of SO2 and NH3 in heterogeneous reactions on the surface of mineral oxides was comprehensively studied. It was revealed that this process is an important pathway for the formation of sulfate and ammonium aerosols in the atmosphere (PCCP, 2016, 18 (2), 956; JPCA 2018, 122 (30), 6311; ESNano, 2019 6, 2749 (Hot paper); AE, 2019, 208: 133);
1.4. NH3 can promote the dissolution of SO2 in aqueous reactions, and the ionic strength can change the rate constant of SO2 oxidation by NO2, and then accelerate the formation of sulfate (EP, 2019, 252, 236);
1.5. The results of analysis of monitoring data obtained during the strong haze in winter in the Beijing area show that the coexistence of mineral particles and high concentrations of NOx during haze episodes are the key factors promoting the conversion of SO2 to sulfate. Under the conditions of combined pollution, the environmental capacity of SO2 decreases, resulting in the rapid growth of sulfate and haze. (SR, 2014 (4): 04172; EP, 2018, 233, 662).

2. Research progress in research on synergistic effects in humidification-dehumidification of mixed particles
2.1. A water vapor adsorption instrument based on the pressure balance method (JPCA, 2010, 114, 4232) and a hygroscopic tandem differential mobility analyzer (HTDMA) (ACS Earth and Space Chemistry, 2019, 3 (7), 1216) were established. Combined with in-situ infrared spectroscopy and Raman spectroscopy, the promotion effect of heterogeneous reaction on the hygroscopicity of particles and its mechanism were revealed (PCCP, 2012, 14 (23), 8403; JES, 2010, 22 (4), 555; 2012, 24 (1), 62);
2.2. The synergistic effect in the multiphase process changed the occurrence form of sulfate in the atmosphere, which was the key mechanism for the formation of secondary gypsum in the atmosphere (PCCP, 2013, 15, 19196);
2.3. It was found that aqueous reactions took place in the water absorption process of organic-inorganic mixed particles. The mechanism of replacing strong acid by weak acid was clarified. (EST, 2013, 47 (18), 10381; AE, 2012, 50, 97; 2013, 69, 281; 2019, 200, 34).
3. Research progress in heterogeneous processes on the soot surface
3.1. Different soot samples were prepared from toluene, n-hexane, and decane combustion under controlled conditions. Based upon chemical and property analysis, it has been found that the hydrophilicity of n-hexane and decane flame soot increased with decreasing fuel/oxygen ratio, while the relationship is inverse for the mean particle size. Fuel/oxygen ratio has little effect on the morphology of aggregates. The hydrophilic functional groups have been found to be mainly located at graphene layer edges and on surface graphene layers in soot. (JPCA, 2012, 116, 4129);
3.2. Heterogeneous reactions of soot toward O3were investigated using in situ Raman spectroscopy. It has been found that the disorder degree of the micro-crystalline carbon in soot particles increased after heterogeneous oxidation. The disordered carbon was identified as the reactive component for the ozonization. The oxygen-containing species on soot were increased during aging by O3, and sunlight could further enhance the heterogeneous oxidation. (PCCP, 2010, 12, 10896; JPC, 2012, 137, 84507; PCCP, 2016, 18, 24401) ;
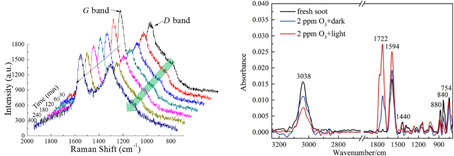
3.3. It has been found that NO2 uptake coefficients and HONO yields decrease with decreasing fuel/oxygen ratio during soot preparation; and OC is the reactive component in soot when reacting with NO2. Sunlight can enhance the reactivity of soot towards NO2 and cause the photolysis of newly-formed nitro compounds, which can become an atmospheric HONO source. (EST, 2013, 47,3174; AE, 2013, 64,270) ;

3.4. It has been found that visible light irradiation greatly promotes the heterogeneous reaction between soot and O2. Various oxygen-containing species (C=O, C-O-C) were formed on soot during photochemical aging. OC dominantly participates in the reaction with O2 under sunlight. (PNAS, 2012, 109, 21250) ;

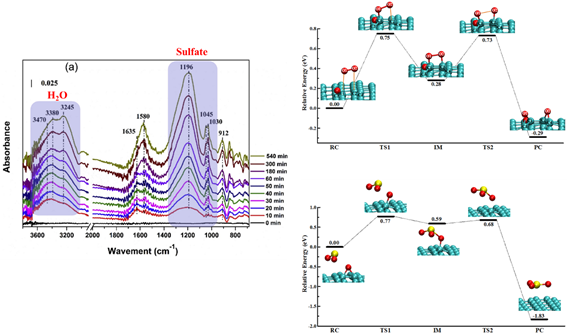
3.5. It has been observed that soot particles can catalyze the oxidation of SO2 by O2 to form sulfate. The catalytic mechanism was revealed by DFT calculation. The ether groups (C-O-C) are the active sites that enable the dissociation of O2 to reactive epoxy groups under ambient conditions. The epoxy groups on soot can easily oxidize SO2. (AE, 2016, 142, 383; PCCP, 2016, 18, 31691; AE, 2017, 152, 465; ACS Catal, 2018, 8, 3825).

53) Biwu Chu, Yali Wang, Weiwei Yang, Jinzhu Ma, Qingxin Ma*, Peng Zhang, Yongchun Liu, Hong He, “Effects of NO2 and C3H6 on the heterogeneous oxidation of SO2 on TiO2 in the presence or absence of UV-Vis irradiation.” Atmos. Chem. Phys., 19, (2019) 14777-14790.
52) Shuping Zhang, Jia Xing*, Golam Sarwar, Yanli Ge, Hong He, Fengkui Duan, Yan Zhao, Kebin He, Lidan Zhu, Biwu Chu*, “Parameterization of heterogeneous reaction of SO2 to sulfate on dust with coexistence of NH3 and NO2 under different humidity conditions”, Atmos. Environ., 208, (2019) 133-140.
51) Qingxin Ma, Cheng Zhong, Chang Liu, Jun Liu, Jinzhu Ma, Lingyan Wu, Hong He*, “A comprehensive study about the hygroscopic behavior of mixtures of oxalic acid and nitrate salts: Implication for the occurrence of atmospheric metal oxalate complex”, ACS Earth. Space Chem., 3, (2019) 1216-1225.
50) Qingxin Ma*, Ling Wang, Biwu Chu, Jinzhu Ma, Hong He*, “Contrary role of H2O and O2 in the kinetics of heterogeneous photochemical reactions of SO2 on TiO2”, J. Phys. Chem. A., 123, (2019) 1311-1318.
49) Qingxin Ma, Chang Liu, Jinzhu Ma, Biwu Chu, Hong He*, “A laboratory study on the hygroscopic behavior of H2C2O4-containing mixed particles”, Atmos. Environ., 200, (2019) 34-39.
48) Tianzeng Chen, Biwu Chu*, Yanli Ge, Shuping Zhang, Qingxin Ma, Hong He, Shao-Meng Li, “Enhancement of aqueous sulfate formation by the coexistence of NO2/NH3 under high ionic strengths in aerosol water”, Environ. Pollut., 252, (2019), 236-244.
47) 王铃,马庆鑫*,贺泓,“光照对SO2在矿质氧化物表面非均相反应的影响”,环境科学,38 (3), (2018) 1-2.
46) Weiwei Yang, Qinxin Ma*, Yongchun Liu, Jinzhu Ma, Biwu Chu, Ling Wang, Hong He, “Role of NH3 in the heterogeneous formation of secondary inorganic aerosols on mineral oxides”, J. Phys. Chem. A, 122, (2018) 6311-6320.
45) Guangzhi He, Jinzhu Ma,Hong He*, “Role of carbonaceous aerosols in catalyzing sulfate formation”, ACS. Catal., 8, (2018) 3825-3832.
44) Jinzhu Ma, Biwu Chu, Jun Liu, Yongchun Liu, Hongxing Zhang,Hong He*, “NOX promotion of SO2 conversion to sulfate: An important mechanism for the occurrence of heavy haze during winter in Beijing”, Environ. Pollut., 233, (2018) 662-669.
43) Chang Liu, Qingxin Ma,Hong He*, Guangzhi He, Jinzhu Ma, Yongchun Liu, Ying Wu, “Structure-activity relationship of surface hydroxyl groups during NO2 adsorption and transformation on TiO2 nanoparticles”, Environ. Sci. Nano., 4, (2017) 2388-2394.
42) Qingxin Ma*, Tao Wang*, Chang Liu, Hong He, Zhe Wang, Weihan Wang, Yutong Liang, “SO2 initiates the efficient conversion of NO2 to HONO on MgO surface”, Environ. Sci. Technol., 51, (2017) 3767-3775.
41) Weiwei Yang, Jianghao Zhang, Qingxin Ma*, Yan Zhao, Yongchun Liu, Hong He*, “Heterogeneous reaction of SO2 on manganese oxides: the effect of crystal structure and relative humidity”, Sci. Rep., 7, (2017) 4550.
40) Chong Han, Yongchun Liu, Hong He*, “Heterogeneous reaction of NO2 with soot at different relative humidity”, Environ. Sci. Pollut. Res., 24, (2017) 21248-21255.
39) Yan Zhao, Yongchun Liu*, Jinzhu Ma, Qingxin Ma, Hong He*, “Heterogeneous reaction of SO2 with soot: the roles of relative humidity and combustion conditions in sulfuric acid formation”, Atmos. Environ., 152, (2017) 465-476.
38) Guangzhi He, Hong He*, “DFT studies on the heterogeneous oxidation of SO2 by oxygen functional groups on grapheme”, Phys. Chem. Chem. Phys., 18, (2016) 31691-31697.
37) Yan Zhao, Qingxin Ma, Yongchun Liu, Hong He*, “Influence of sulfur in fuel on the properties of diffusion flame soot”, Atmos. Environ., 142, (2016) 383-392.
36) Chong Han, Yongchun Liu, Hong He*, “The photoenhanced aging process of soot by the heterogeneous ozonization reaction”, Phys. Chem. Chem. Phys., 18, (2016) 24401-24407.
35) Weiwei Yang , Hong He* , Qingxin Ma , Jinzhu Ma , Yongchun Liu , Pengfei Liu, Yujing Mu, “Synergistic formation of sulfate and ammonium resulting from reaction between SO2 and NH3 on typical mineral dust”, Phys. Chem. Chem. Phys., 18, (2016) 956-964.
34) Biwu Chu, Tengyu Liu, Xiao Zhang, Yongchun Liu, Qingxin Ma, Jinzhu Ma, Hong He*, Xinming Wang, Junhua Li, Jiming Hao, “Secondary aerosol formation and oxidation capacity in photooxidation in the presence of Al2O3 seed particles and SO2”, Sci. China-Chem., 58 (9), (2015) 1426-1434.
33) 马庆鑫,马金珠,楚碧武,刘永春,赖承钺,贺泓*,“矿质和黑碳颗粒物表面大气非均相反应研究进展”, 科学通报,60(2),(2015)122-136.
32) Yongchun Liu, Chong Han, Jinzhu Ma, Xiaolei Bao, Hong He*, “Influence of relative humidity on heterogeneous kinetics of NO2 on kaolin and hematite”, Phys. Chem. Chem. Phys., 17, (2015) 19424-19431.
31) Chengyue Lai, Yongchun Liu*, Jinzhu Ma, Qingxin Ma, Biwu Chu, and Hong He*, “Heterogeneous kinetics of cis-pinonic acid with hydroxyl radical under different environmental conditions”, J. Phys. Chem. A, 119, (2015) 6583-6593.
30) Chengyue Lai, Yongchun Liu*, Jinzhu Ma, Qingxin Ma, Hong He*, “Laboratory study on OH-initiated degradation kinetics of dehydroabietic acid”, Phys. Chem. Chem. Phys., 17, (2015) 10953-10962.
29) Chengyue Lai, Yongchun Liu*, Jinzhu Ma, Qingxin Ma, Hong He*, “Degradation kinetics of levoglucosan initiated by hydroxyl radical under different environmental conditions”, Atmos. Environ., 91, (2014) 32-39.
28) Biwu Chu, Kun Wang, Hideto Takekawa, Junhua Li, Wei Zhou, Jingkun Jiang, Qingxin Ma, Hong He, Jinming Hao*, “Hygroscopicity of particles generated from photooxidation of α-pinene under different oxidation conditions in the presence of sulfate seed aerosols”, J. Environ. Sci., 26, (2014) 129-139.
27) Hong He*, Yuesi Wang*, Qingxin Ma, Jinzhu Ma, Biwu Chu, Dongsheng Ji, Guiqian Tang, Chang Liu, Hongxing Zhang, Jiming Hao, “Mineral dust and NOx promote the conversion of SO2 to sulfate in heavy pollution days”, Sci. Rep., 4, (2014) 04172.
26) Qingxin Ma, Hong He*, Yongchun Liu, Chang Liu, Vicki H. Grassian, “Heterogeneous and multiphase formation pathways of gypsum in the atmosphere”, Phys. Chem. Chem. Phys., 15, (2013) 19196-19204.
25) Qingxin Ma, Jinzhu Ma, Chang Liu, Chengyue Lai, Hong He*, “Laboratory study on the hygroscopicbehavior of external and internal C2−C4 dicarboxylic Acid−NaCl mixtures”, Environ. Sci. Technol., 47, (2013) 10381-10388.
24) Qingxin Ma, Hong He*, Chang Liu, “Hygroscopic properties of oxalic acid and atmospherically relevant oxalates”, Atmos. Environ., 69, (2013) 281-288.
23) Chong Han, Yongchun Liu, Hong He*, “Role of Organic Carbon in Heterogeneous Reaction of NO2 with Soot”, Environ. Sci. Technol., 47, (2013) 3174-3181.
22) Jinzhu Ma, Yongchun Liu, Chong Han, Qingxin Ma, Chang Liu, Hong He*, “Review of heterogeneous photochemical reactions of NOy on aerosol – A possible daytime source of nitrous acid (HONO) in the atmosphere”, J. Environ. Sci., 25, (2013) 326-334.
21) Jinzhu Ma, Yongchun Liu, Qingxin Ma, Chang Liu, Hong He*,“Heterogeneous photochemical reaction of ozone with anthracene adsorbed on mineral dust”, Atmos. Environ., 72, (2013) 165-170.
20) Chong Han, Yongchun Liu*, Hong He*, “Heterogeneous photochemical aging of soot by NO2 under simulated sunlight”, Atmos. Environ., 64, (2013) 270-276.
19) Chong Han, Yongchun Liu*, Jinzhu Ma, Hong He*, “Key role of organic carbon in the sunlight enhanced atmospheric aging of soot by O2”, Proc. Nat. Acad. Sci. USA., 109(52), (2012) 21250-21255.
18) Yongchun Liu, Qingxin Ma, Hong He*, “Heterogeneous Uptake of Amines by Citric Acid and Humic Acid”, Environ. Sci. Technol., 46, (2012) 11112-11118.
17) Chong Han, Yongchun Liu*, Jinzhu Ma, Hong He*, “Effect of soot microstructure on its ozonization reactivity”, J. Chem. Phys., 137, (2012) 084507.
16) Yongchun Liu, Chong Han, Chang Liu, Jinzhu Ma, Qingxin Ma, Hong He*, “Differences in the reactivity of ammonium salts with methylamine”, Atmos. Chem. Phys., 12, (2012) 4855-4865.
15) Qingxin Ma, Yongchun Liu, Chang Liu, Hong He*, “Heterogeneous reaction of acetic acid on MgO, a-Al2O3, and CaCO3 and the effect on the hygroscopic behaviour of these particles”, Phys. Chem. Chem. Phys., 14, (2012) 8403-8409.
14)Chong Han, Yongchun Liu*, Chang Liu, Jinzhu Ma, Hong He*, “Influence of combustion conditions on hydrophilic properties and microstructure of flame soot”, J. Phys. Chem. A, 116, (2012) 4129-4136.
13) Qingxin Ma, Yongchu Liu, Chang Liu, Jinzhu Ma, Hong He*, “A case study of Asian dust storm particles: Chemical composition, reactivity to SO2 and hygroscopic properties”, J. Environ. Sci, 24, (2012) 62-71.
12) Qingxin Ma, Hong He*, “Synergistic effect in the humidifying process of atmospheric relevant calcium nitrate, calcite and oxalic acid mixtures”, Atmos. Environ., 50, (2012) 97-102.
11) Chang Liu, Qingxin Ma, Yongchun Liu, Jinzhu Ma, Hong He*, “Synergistic reaction between SO2 and NO2 on mineral oxides: a potential formation pathway of sulfate aerosol. Phys. Chem. Chem. Phys., 14, (2012) 1668-1676.
10) 马金珠、刘永春、马庆鑫、刘畅、贺泓*, “大气非均相反应及其环境效应”, 环境化学, 30, (2011) 97-119.
9) Jinzhu Ma, Yongchun Liu, Hong He*, “Heterogeneous reactions between NO2 and anthracene adsorbed on SiO2 and MgO ”, Atmos. Environ., 45, (2011) 917-924.
8) Jinzhu Ma, Yongchun Liu, Hong He*, “Degradation kinetics of anthracene by ozone on mineral oxides”, Atmos. Environ., 44, (2010) 4446-4453.
7) Yongchun Liu, Chang Liu, Jinzhu Ma, Qingxin Ma, Hong He*, “Structural and hygroscopic changes of soot during heterogeneous reaction with O3”, Phys. Chem. Chem. Phys., 12, (2010) 10896-10903.
6) Qingxin Ma, Yongchun Liu, Chang Liu, Jinzhu Ma, Hong He*, “A comprehensive characterisation of Asian dust storm particles: chemical composition, reactivity to SO2, and hygroscopic property”, Atmos. Chem. Phys. Discuss., 10, (2010) 8899-8925.
5) Qingxin Ma, Hong He*,Yongchun Liu, “In situ DRIFTS study of hygroscopic behavior of mineral aerosol ”, J. Environ. Sci., 22, (2010) 555-560.
4)Qingxin Ma, Yongchun Liu, Hong He*, “The utilization of physisorption analyzer for studying the hygroscopic properties of atmospheric relevant particles”, J. Phys. Chem. A, 114, (2010) 4232-4237.
3) Yongchun Liu, Qingxin Ma, Hong He*, “Comparative study of the effect of water on the heterogeneous reactions of carbonyl sulfide on the surface of α-Al2O3 and MgO” , Atmos. Chem. Phys., 9, (2009) 6273-6286.
2) 贺泓*, 刘永春,曲久辉, “环境微界面过程的原位和在线研究方法”, 环境科学学报,29, (2009) 11-20.
1) Qingxin Ma, Yingchun Liu, Hong He*, “Synergistic effect between NO2 and SO2 in their adsorption and reaction on γ-Alumina”, J. Phys. Chem. A, 112, (2008) 6630-6635.
Group of Environmental Catalysis and Green Chemistry

You are the 1854288th visitor
Copyright Professor Hong He's Group 2019